Table 1 Structures and anti-α-glucosidase activities (IC50 values, µM) of indole-carbohydrazide-phenoxy-1,2,3-triazole-N-phenylacetamide derivatives 11a–o 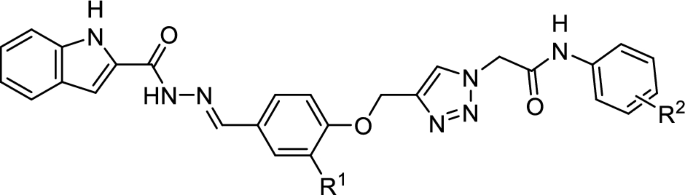

Compound | R1 | R2 | IC50 (µM) |
|---|---|---|---|
11a | H | H | 15.21 ± 0.18 |
11b | H | 2-CH3 | 8.88 ± 0.07 |
11c | H | 4-CH3 | 19.12 ± 0.08 |
11d | H | 4-OCH3 | 6.31 ± 0.03 |
11e | H | 2-Cl | 26.97 ± 0.25 |
11f | H | 4-Cl | 9.53 ± 0.12 |
11g | H | 4-Br | 49.89 ± 0.09 |
11h | H | 4-NO2-3-CH3 | 35.11 ± 0.07 |
11i | OCH3 | H | 11.25 ± 0.15 |
11j | OCH3 | 4-OCH3 | 24.19 ± 0.1 |
11k | OCH3 | 4-Cl | 31.56 ± 0.12 |
11l | OCH3 | 2,3-Dichloro | 18.2 ± 0.81 |
11m | OCH3 | 3,5-Dichloro | 14.2 ± 0.21 |
11n | OCH3 | 4-Br | 8.3 ± 0.16 |
11o | OCH3 | 2-Cl-3-NO2 | 12.67 ± 0.05 |
Acarbose | – | - | 750.0 ± 10.0 |