Table 1 The antiproliferative activities of the designed compounds (4a-4l)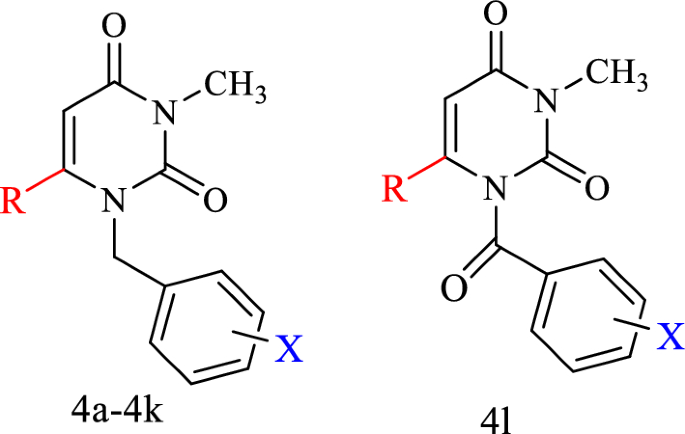

IC50 ± SD (µM) | IC50 ± SD (µM) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Entry | R | X | Entry | R | X | ||||||
MCF-7 | HEPG-2 | MRC-5 | MCF-7 | HEPG-2 | MRC-5 | ||||||
4a | Imidazole | H | 150.5 ± 0.77 | 193.3 ± 7.98 | > 200 | 4g | 2-Me-Imidazole | 4-Br | 109.2 ± 3.74 | 105.65 ± 3.03 | > 200 |
4b | Imidazole | 3-Me | 17.12 ± 1.30 | 48.47 ± 1.97 | 61.8 ± 2.30 | 4h | Triazole | H | 131.4 ± 2.14 | 108.15 ± 10.81 | > 200 |
4c | Imidazole | 4-Me | 26.13 ± 4.41 | 27.18 ± 1.66 | 101.3 ± 3.02 | 4i | Triazole | 3-Me | 43.1 ± 2.07 | 65.40 ± 2.15 | > 200 |
4d | Imidazole | 4-Br | 130.63 ± 3.1 | 177.4 ± 4.06 | > 200 | 4j | Triazole | 4-Me | 16.18 ± 1.02 | 7.56 ± 5.28 | 57.3 ± 2.08 |
4e | 2-Me-Imidazole | H | 91.95 ± 2.19 | ND | > 200 | 4k | Triazole | 4-Br | 78.15 ± 4.64 | 56.42 ± 2.46 | > 200 |
4f | 2-Me-Imidazole | 4-Me | 65.45 ± 3.1 | 55.85 ± 6.58 | > 200 | 4l | Triazole | H | 67.55 ± 2.41 | 72.8 ± 2.19 | > 200 |
Cis platin | – | 20.70 ± 0.83 | 15.91 ± 1.83 | 45.2 ± 2.5 | |||||||